Neuren Pharmaceuticals (ASX: NEU) has delivered news that could reshape investor expectations for the Melbourne-based biotech company. The firm’s partner, Acadia Pharmaceuticals, presented updated projections at a major healthcare conference that signal strong commercial momentum ahead.
The headline number is significant. DAYBUE global net sales are now projected to reach approximately US$700 million by 2028. This represents substantial growth from current levels and reflects confidence in the drug’s expanding market presence.
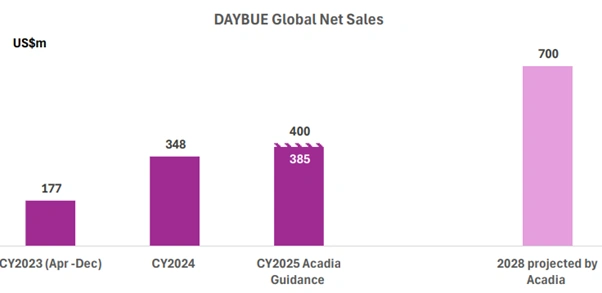
Figure 1: DAYBUE global net sales history and Acadia Pharmaceuticals’ projected revenue outlook through 2028. [Source: Market Index]
What makes this projection particularly noteworthy is the breadth of growth drivers supporting it. Neuren Pharmaceuticals is not relying on a single catalyst but rather a combination of product innovation, market expansion, and increasing patient adoption across multiple geographies.
Neuren Pharmaceuticals Sees Multiple Growth Drivers Align
Neuren Pharmaceuticals received the updated forecast during Acadia Pharmaceuticals’ presentation at the 44th annual J.P. Morgan Healthcare Conference. The partner company outlined three key factors expected to drive DAYBUE’s path to the revenue target.
The first driver is the rollout of DAYBUE STIX, a new powder formulation approved by the US Food and Drug Administration in December 2025. This alternative format could potentially enable patient growth from families who had declined to try or discontinued the liquid formulation. The limited rollout commenced in Q1 2026, with full availability expected by the beginning of Q2 2026.

Figure 2: Neuren Pharmaceuticals corporate branding used in official communications and investor materials. [Source: PWSA USA]
Second, Neuren Pharmaceuticals stock is benefiting from continued momentum following the expansion of Acadia’s US customer-facing teams in Q2 2025. The third driver is international expansion, with a Committee for Medicinal Products for Human Use opinion anticipated in Q1 2026 for European approval.
Since DAYBUE’s launch in the US in 2023, more than 2,000 Rett syndrome patients have been treated with the therapy. The 12-month persistency rate has now increased to 55 per cent, indicating more than half of patients continue using it after a full year.
The diagnosed patient population in the US has also expanded to approximately 6,000 patients, up 30 per cent since launch from the previously estimated range of 5,500 to 5,800. Over 300 patients are participating in Acadia’s real-world LOTUS study, which continues to develop and publish data.
International Expansion Progresses Across Key Markets
Neuren Pharmaceuticals is advancing its geographic footprint beyond the initial US launch. Acadia announced that DAYBUE oral solution has been approved by the Ministry of Health in Israel, marking another market entry for the treatment.
In Japan, the Phase 3 clinical trial of trofinetide is ongoing. Top-line results are expected between Q4 2026 and Q1 2027. A successful outcome would support regulatory submissions in one of the world’s largest pharmaceutical markets.

Figure 3: Pharmaceutical laboratory setting illustrating drug research, development, and formulation activities. [Source: Freepik]
The European approval pathway represents the most significant near-term international opportunity. With a Committee for Medicinal Products for Human Use opinion anticipated in Q1 2026, Neuren Pharmaceuticals ASX could soon gain access to the European Union’s substantial patient population.
Pipeline Development Adds Long-Term Value Potential
Beyond DAYBUE, Neuren Pharmaceuticals is developing NNZ-2591 for multiple neurodevelopmental disorders. The Company has achieved positive results in Phase 2 clinical trials across three rare conditions: Phelan-McDermid syndrome, Pitt-Hopkins syndrome, and Angelman syndrome.
Each programme has been granted orphan drug designation in both the United States and the European Union. This provides incentives, including potential market exclusivity periods and regulatory fee waivers.
These pipeline assets represent additional value drivers for Neuren Pharmaceuticals stock beyond the current DAYBUE commercialisation. Success in any of these indications could create meaningful additional revenue streams and diversify the Company’s commercial portfolio.
Neuren Pharmaceuticals Stock Trades Near Multi-Year Highs
Neuren Pharmaceuticals ASX stock closed at $20.350, giving the Company a market capitalisation of $2.44 billion. The shares have traded in a 52-week range of $8.610 to $22.985 per share, with the current price sitting near the upper end.
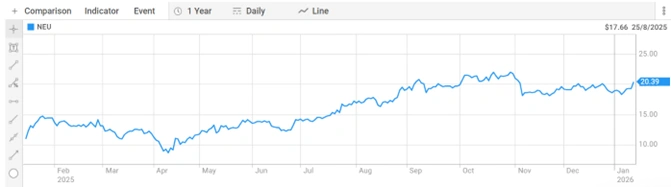
Figure 4: Neuren Pharmaceuticals 52-week share price chart. [Source: ASX]
Final Thoughts
Neuren Pharmaceuticals has reached an important milestone with its partner’s projection that DAYBUE global net sales will reach approximately US$700 million by 2028. This forecast reflects confidence in multiple growth drivers including new formulations, international expansion, and continued US market penetration.
The Company’s execution since DAYBUE’s 2023 US launch has been solid. More than 2,000 patients treated, increasing persistency rates to 55 per cent, and a growing diagnosed patient population all point to meaningful commercial traction. The DAYBUE STIX formulation addresses a specific market need and could unlock patient segments that were previously inaccessible.
Beyond DAYBUE, the NNZ-2591 pipeline offers additional long-term value creation potential. Positive Phase 2 results across multiple rare neurodevelopmental disorders position Neuren Pharmaceuticals to potentially replicate its DAYBUE success in additional indications.
Also Read: Cosmic Crash Sparks Mysterious Black Hole Birth in Infinity Galaxy
FAQs
Q1. What revenue target has been set for DAYBUE by 2028?
Ans. Acadia Pharmaceuticals projected that DAYBUE global net sales will reach approximately US$700 million by 2028, representing substantial growth from current levels for Neuren Pharmaceuticals.
Q2. How many patients have been treated with DAYBUE since launch?
Ans. More than 2,000 Rett syndrome patients have been treated with DAYBUE since its US launch in 2023, with 12-month persistency rates now reaching 55 per cent.
Q3. What is DAYBUE STIX and when will it be available?
Ans. DAYBUE STIX is a powder formulation approved by the US FDA in December 2025. Neuren Pharmaceuticals partner Acadia began a limited rollout in Q1 2026, with full availability expected by beginning of Q2 2026.
Q4. Where else is DAYBUE being approved internationally?
Ans. DAYBUE oral solution has been approved by the Ministry of Health in Israel. Neuren Pharmaceuticals ASX is also expecting a Committee for Medicinal Products for Human Use opinion in Q1 2026 for European approval, with Japan Phase 3 top-line data expected Q4 2026 to Q1 2027.