Neuren Pharmaceuticals Limited (ASX: NEU) has announced that the United States Congress has reauthorised the Rare Pediatric Disease Priority Review Voucher (PRV) program, a development that supports the commercial framework underpinning rare disease drug approvals. The update is strategically relevant to Neuren’s portfolio, including trofinetide, which targets Rett syndrome.

Neuren Pharmaceuticals focuses on developing therapies for rare neurodevelopmental disorders, including Rett syndrome.(Source: StockWire X)
The reauthorisation ensures the continuation of a regulatory incentive mechanism that enables eligible companies to receive a transferable Priority Review Voucher upon approval of a qualifying rare paediatric disease therapy. Such vouchers can significantly accelerate the US Food and Drug Administration (FDA) review timeline for another product or be sold to third parties.
For Neuren, the reinstatement of the PRV program reinforces regulatory and commercial optionality within the US market, the world’s largest pharmaceutical market by value.
Key Regulatory Development
The Priority Review Voucher program was designed to incentivise the development of therapies for rare paediatric diseases by offering accelerated review timelines for future drug applications.
Key elements of the reauthorised program include:
- Issuance of a transferable Priority Review Voucher upon approval of eligible rare paediatric disease therapies
- Potential reduction of FDA review time from standard review (approximately 10 months) to priority review (approximately six months)
- Ability to transfer or sell the voucher to another company
- Continued support for innovation in underserved paediatric conditions
Historically, PRVs have commanded substantial value in secondary market transactions, reflecting the commercial advantage of accelerated FDA review. In previous industry transactions, vouchers have been sold for amounts ranging from tens to hundreds of millions of US dollars, depending on market conditions.

The US FDA’s Priority Review Voucher program aims to accelerate approval timelines for qualifying therapies. (Source: iStock)
The reinstatement restores certainty around this incentive structure and aligns with broader US policy objectives to stimulate rare disease drug development.
Strategic Significance for Neuren
Neuren Pharmaceuticals is focused on developing therapies for neurodevelopmental disorders. Its lead product, trofinetide, is approved in the United States for the treatment of Rett syndrome, a rare genetic neurological disorder.
The continuation of the PRV program provides a supportive regulatory environment for rare disease innovators, reinforcing incentives for ongoing research and development investment.
While the announcement does not alter Neuren’s existing approvals or development timelines, it strengthens the broader commercial framework applicable to rare paediatric disease therapies. The PRV mechanism has historically enhanced valuation potential for qualifying assets, particularly where vouchers can be monetised or used to accelerate future pipeline candidates.

Regulatory incentives are designed to encourage the development of treatments for rare paediatric conditions. (Source: pedpartners)
The global rare disease therapeutics market continues to expand, driven by improved genetic diagnostics, regulatory incentives and increasing investment in precision medicine. The United States remains the largest market for orphan drug approvals, supported by both market exclusivity provisions and incentive programs such as PRVs.
Pipeline and Development Context
Neuren’s development strategy centres on addressing significant unmet needs in neurodevelopmental disorders. Trofinetide represents a milestone in Rett syndrome treatment, and the company continues to evaluate additional indications and life-cycle management opportunities.
Clinical development in rare paediatric disorders typically involves:
- Targeted patient populations
- Orphan drug designation pathways
- Engagement with regulatory authorities under accelerated review frameworks
- Ongoing post-marketing safety monitoring
Rare disease drug development often benefits from regulatory support measures, including priority review, orphan drug exclusivity and fee waivers. These frameworks aim to offset the higher per-patient research costs associated with small patient populations.
Environmental, social and governance (ESG) considerations are increasingly relevant in biopharmaceutical development, particularly in ensuring ethical clinical trial conduct, patient access programs and transparent regulatory engagement.
Market and Industry Context
Neuren Pharmaceuticals shares last traded at $13.200, down $1.429 (-9.774%), with 849,294 shares exchanged. The company has a market capitalisation of approximately $1.85 billion, positioning it within the mid-cap segment of the ASX healthcare sector.
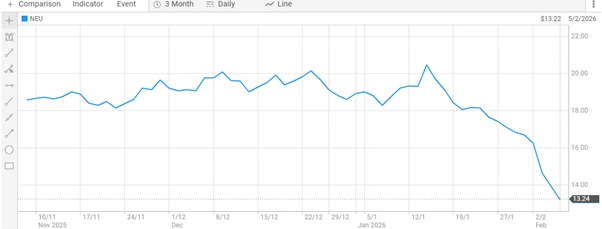
Neuren Pharmaceuticals Share Price [ASX]
Share price volatility in biotechnology stocks is common, particularly where valuations are influenced by regulatory developments, clinical milestones and commercial performance metrics. The reauthorisation of the PRV program represents a supportive policy development rather than a direct operational milestone.
Across the broader biotechnology industry, regulatory incentives remain central to investment decisions in rare disease programs. The reinstatement of the PRV program may contribute to improved sentiment across companies focused on paediatric and orphan indications.
For investors and analysts, the key considerations remain commercial uptake of approved therapies, pipeline progression and capital allocation discipline. Regulatory clarity in major markets such as the United States supports long-term strategic planning and valuation frameworks.
Also Read: Telix Pharmaceuticals Shares in Focus After FY25 Results & Investor Webcast Update
Outlook
The reauthorisation of the Rare Pediatric Disease Priority Review Voucher program provides renewed regulatory certainty for companies developing treatments in underserved paediatric populations. For Neuren Pharmaceuticals, the continuation of this framework reinforces the commercial ecosystem surrounding rare disease innovation.
As the company advances its neurodevelopmental portfolio, investors will monitor regulatory developments, commercial performance metrics and pipeline expansion initiatives. The sustained availability of incentive mechanisms such as PRVs remains a constructive feature of the US rare disease regulatory landscape.
FAQs
- What is a Priority Review Voucher (PRV)?
A Priority Review Voucher is a regulatory incentive issued by the US Food and Drug Administration (FDA) to companies that gain approval for eligible rare paediatric disease treatments. The voucher allows the holder to receive priority review for another drug application or sell the voucher to another company.
- What does priority review mean?
Priority review reduces the FDA’s review timeline for a new drug application from the standard approximately 10 months to around six months. This accelerated process can bring therapies to market sooner.
- Why did US Congress reauthorise the PRV program?
The PRV program was reauthorised to continue incentivising pharmaceutical companies to develop treatments for rare paediatric diseases. These conditions often affect small patient populations and may not attract sufficient commercial investment without regulatory incentives.
- How does the PRV program benefit Neuren Pharmaceuticals?
The continuation of the PRV program supports the commercial framework for rare disease therapies such as trofinetide, Neuren’s treatment for Rett syndrome. It enhances regulatory certainty and maintains incentives that can add strategic value to qualifying therapies.
- Can Priority Review Vouchers be sold?
Yes. PRVs are transferable and can be sold to other pharmaceutical companies. Historically, vouchers have been sold for substantial amounts, reflecting the commercial value of accelerated FDA review.
- What is trofinetide used for?
Trofinetide is approved in the United States for the treatment of Rett syndrome, a rare genetic neurodevelopmental disorder that primarily affects girls.
- What is a rare paediatric disease?
A rare paediatric disease is a serious or life-threatening condition that primarily affects individuals aged 18 years or younger and impacts a small patient population, as defined under US regulatory criteria.
- Why are regulatory incentives important in rare disease drug development?
Rare diseases often involve limited patient populations, making research and development costly relative to potential revenue. Incentive programs such as orphan drug designation and PRVs help offset financial risk and encourage innovation.