Shoppers across the United States and Puerto Rico are rushing to check their medicine cabinets after Walmart and other major retailers pulled popular hand soap products from shelves. The urgent recall, announced by the Food and Drug Administration affects thousands of bottles sold nationwide due to dangerous bacterial contamination.
Four Products Recalled Over Sepsis Fears
DermaRite Industries has voluntarily recalled several healthcare products after testing revealed contamination with Burkholderia cepacia, a bacterium that can cause life-threatening infections. The affected products include:
- DermaKleen Antimicrobial Lotion Soap – antiseptic lotion soap with Vitamin E
- KleenFoam Antimicrobial Foam Soap – antibacterial foam cleanser
- PeriGiene Antiseptic Cleanser – healthcare-grade antiseptic
- DermaSarra External Analgesic Lotion – topical pain relief with skin cleansing properties
These products were distributed nationwide through healthcare suppliers and major retailers including Walmart, both in physical stores and online platforms.
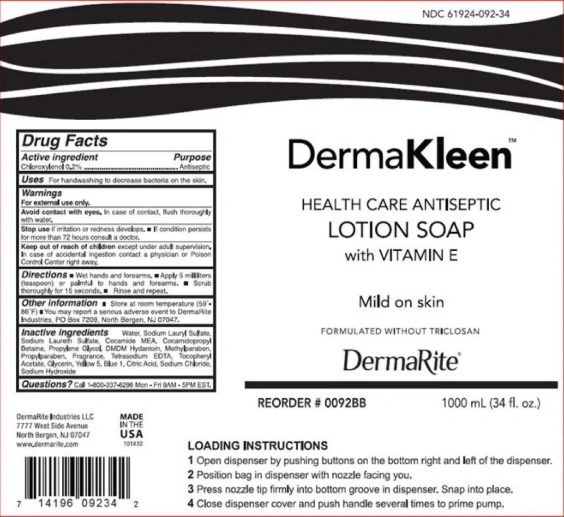
DermaKleen Antimicrobial Lotion Soap
The Hidden Danger in Your Soap Dispenser
Burkholderia cepacia complex represents a group of bacteria commonly found in soil and water environments. According to the Centers for Disease Control and Prevention, these organisms pose minimal risk to healthy individuals but can trigger severe complications in vulnerable populations.
“People who have certain health problems like weakened immune systems or chronic lung diseases, particularly cystic fibrosis, may be more susceptible to B. cepacia infections,” the CDC warned in its safety notice.
The most alarming concern involves immunocompromised individuals, where the infection can spread into the bloodstream and cause sepsis – a potentially fatal immune system response. Healthcare workers and family members caring for vulnerable patients face elevated exposure risks through routine handwashing with contaminated products.
Also Read: Trump Enacts First-Ever Federal Takeover of DC Police Under Emergency Powers
Check Your Products Immediately
Consumers should examine their soap bottles for specific lot numbers and expiration dates. The recall affects products with expiration dates spanning from August 2025 through February 2027. Complete details including lot numbers are available on the FDA’s official recall database.
Mary Goldberg from DermaRite Industries confirmed the company has received no illness reports related to the contaminated products. However, she stressed the importance of immediate action: “We initiated this recall out of an abundance of caution to ensure customer safety.”
What Shoppers Must Do Now
Health authorities have issued clear instructions for anyone who purchased these products:
- Stop using recalled items immediately – do not continue using even if no symptoms appear
- Dispose safely – avoid pouring contents down drains where bacteria could spread
- Monitor for symptoms including fever, fatigue, unusual skin irritation, or respiratory issues
- Seek medical attention if you develop signs of infection, particularly if immunocompromised
- Report adverse effects through the FDA’s MedWatch system
Customers can return products to their point of purchase for full refunds. Those with questions can contact DermaRite directly at 973-569-9000 extension 104 during business hours or email [email protected].
Walmart’s Response and Industry Impact
Walmart spokesperson confirmed the retailer is working closely with the FDA to remove affected products from shelves and notify customers who purchased them online. The company’s swift response mirrors actions taken during previous contamination incidents affecting major retailers.

This recall joins a concerning pattern of product safety issues affecting Australian and international consumers. Recent months have seen major supermarket chains recall contaminated salads due to E. coli risks and ice cream products pulled over plastic contamination.
DermaRite has instructed all suppliers and distributors to destroy remaining contaminated inventory that hasn’t reached consumers. The company manufactures products primarily in facilities outside the United States, raising questions about international quality control standards.
Broader Safety Implications
Healthcare facilities rely heavily on these antimicrobial products for infection control. The contamination discovery highlights vulnerabilities in supply chain safety monitoring, particularly for products used in medical settings where patients have compromised immune systems.
Consumer advocacy groups are calling for enhanced testing protocols for healthcare products. The incident underscores why product safety recalls require immediate public attention, regardless of whether illnesses have been reported.
Industry experts note that Burkholderia cepacia can survive in moisture-rich environments, making liquid soap products particularly susceptible. Regular quality testing and proper storage conditions remain critical for preventing future contamination incidents.
Prevention and Consumer Awareness
This recall demonstrates the importance of regularly checking product safety alerts. Consumers should:
- Register for FDA recall notifications
- Check product lot numbers against official recall lists
- Monitor vulnerable family members for infection symptoms
- Maintain awareness of ongoing safety investigations
The FDA continues monitoring the situation and may expand the recall if additional contaminated lots are discovered. Healthcare providers have been notified to watch for related infections among patients who may have used these products.
For the latest updates on this developing story and other consumer safety alerts, visit the official Walmart recall page or the FDA’s consumer safety database.