Telix Pharmaceuticals Limited (ASX: TLX) has secured a significant milestone in its China expansion strategy. The Company announced on 20 January 2026 that China’s National Medical Products Administration accepted its New Drug Application for Illuccix. ThisTelix Pharmaceuticals China drug approval news marks the first regulatory filing for any of the Company’s products in the Chinese market.

Figure 1: Telix Pharmaceuticals corporate facility supporting global radiopharmaceutical operations. [Telix Pharmaceuticals]
Telix Pharmaceuticals submitted the application through its strategic partner Grand Pharmaceutical Group Limited. The filing seeks approval for TLX591-Px, marketed as Illuccix, which is a prostate cancer imaging agent. The submission includes data from the China Pivotal Phase 3 Registration study that reported positive results in December 2025.
Study Results Support Regulatory Submission
Telix Pharmaceuticals stock benefited from strong clinical trial results supporting the application. The China study met its primary endpoint with an overall positive predictive value of 94.8 per cent. The trial focused on detecting tumours in patients with biochemical recurrence of prostate cancer.
The results confirmed that TLX591-Px PSMA-PET imaging performs similarly in Chinese patients compared to non-Chinese populations. The high predictive value remained consistent even in patients with very low PSA values. More than two-thirds of patients, specifically 67.2 per cent, experienced changes to their treatment plans following the imaging procedure.
Growing China Market Creates Expansion Opportunity
Telix Pharmaceuticals China drug approval news expansion targets a rapidly growing market with significant unmet needs. More than 134,000 men received prostate cancer diagnoses in China during 2022. The incidence rate increases by approximately 6 per cent each year.
Telix Pharmaceuticals CEO of Precision Medicine, Kevin Richardson, emphasised the strategic importance of this milestone. He stated that submitting this application represents a major achievement for the Company and Grand Pharma. Richardson noted that geographic expansion forms a core component of the Company’s precision medicine business growth strategy.

Figure 2: Telix Pharmaceuticals executive leadership associated with the Company’s precision medicine strategy. [Telix Pharmaceuticals]
The Chinese government supports wider access to nuclear medicine infrastructure. China’s installed base of PET/CT cameras was expected to surpass 1,600 units by the end of 2025. This compares to just 133 cameras in 2010, demonstrating rapid infrastructure development.
Illuccix Already Approved In Multiple Markets
Telix Pharmaceuticals has built a substantial international presence with Illuccix approvals across numerous jurisdictions. The imaging agent received regulatory approval from the U.S. Food and Drug Administration in December 2021. Australian Therapeutic Goods Administration approval followed in November 2021.

Figure 3: Illuccix, Telix Pharmaceuticals’ PSMA-PET imaging agent for prostate cancer detection. [Telix Pharmaceuticals]
Additional approvals came from Health Canada in October 2022 and Brazil’s ANVISA in March 2025. The United Kingdom’s Medicines and Healthcare Products Regulatory Agency granted approval in February 2025. European approvals cover 19 countries within the European Economic Area.
PSMA-PET imaging represents a significant advancement in prostate cancer management compared to conventional methods. The technology largely replaces bone scans and computed tomography as the standard of care. Global guidelines recognise its superior accuracy for staging primary disease and assessing biochemical recurrence.
China Filing Marks First Product Submission
This Telix Pharmaceuticals China drug approval news confirms that the Illuccix application represents the Company’s first product filing in China. Telix Pharmaceuticals is working with Grand Pharmaceutical Group Limited to navigate the Chinese regulatory landscape. The partnership provides local expertise and market access for the Greater China region.
Richardson stated that the Company looks forward to progressing regulatory approvals with Grand Pharma. Subject to NMPA approval, Telix Pharmaceuticals aims to bring its lead commercial imaging product to Chinese patients. The Company sees this as serving the needs of men living with prostate cancer in a strategically important market.

Figure 4: China’s National Medical Products Administration (NMPA), the regulator reviewing Telix Pharmaceuticals’ Illuccix application. [Johner Institute]
The submission seeks a broad label reflecting clinical utility at multiple stages of prostate cancer care. This approach mirrors regulatory strategies in other markets where Illuccix received approval.
Nuclear Medicine Infrastructure Supports Adoption
Telix Pharmaceuticals China drug approval news strategy aligns with expanding nuclear medicine capabilities across the country. Government policy actively supports greater geographic access to advanced imaging technologies. The growth in PET/CT camera installations creates favourable conditions for product adoption.
The infrastructure expansion enables more hospitals and imaging centres to offer PSMA-PET imaging services. This broader accessibility increases the potential patient population that could benefit from Illuccix following regulatory approval.
Telix Pharmaceuticals operates manufacturing and distribution facilities across multiple regions. The Company maintains 38 manufacturing and distribution sites globally as of 30 June 2025. This network positions the Company to support supply requirements in new markets, including China.
Share Price Performance Reflects Market Response
Telix Pharmaceuticals stock closed at $11.43 on 20 January 2026. The shares trade within a 52-week range of $10.885 to $31.970 per share. The Company maintains a market capitalisation of $3.82 billion.
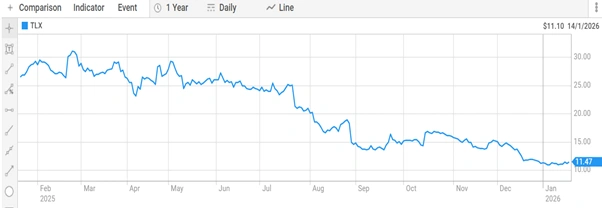
Figure 5: Telix Pharmaceuticals share price performance on the Australian Securities Exchange (ASX: TLX). [ASX]
Investors will monitor regulatory progress in China alongside commercial performance in existing markets. Telix Pharmaceuticals reported strong revenue growth in the recent period, driven by Illuccix sales. The Company generated US$390.4 million in revenue during the first half of 2025, representing 63 per cent growth year-over-year.
China approval could provide additional revenue diversification and support long-term growth objectives. The Company targets revenue between US$770 million and US$800 million for the full 2025 financial year.
About Telix Pharmaceuticals Limited
Telix Pharmaceuticals Limited is a biopharmaceutical company focused on therapeutic and diagnostic radiopharmaceuticals. The Company develops products addressing unmet medical needs in oncology and rare diseases.
Telix Pharmaceuticals maintains its headquarters in Melbourne, Australia, with international operations across the United States, the United Kingdom, Brazil, Canada, Europe and Japan. The Company employs 1,149 people globally as of 30 June 2025. Telix Pharmaceuticals is listed on the Australian Securities Exchange and the Nasdaq Global Select Market under the ticker TLX.
FAQ
Q1. What did China approve for Telix Pharmaceuticals?
Ans. China’s National Medical Products Administration accepted Telix Pharmaceuticals’ New Drug Application for Illuccix, a prostate cancer imaging agent.
Q2. What were the clinical trial results for Illuccix in China?
Ans. The China study achieved a 94.8 per cent positive predictive value and changed treatment plans for 67.2 per cent of patients.
Q3. How many countries have approved Illuccix?
Ans. Telix Pharmaceuticals has secured Illuccix approvals in 23 countries, including the U.S., Australia, Canada, Brazil, the UK and 19 European nations.
Q4. Who is Telix Pharmaceuticals’ partner in China?
Ans. Telix Pharmaceuticals partnered with Grand Pharmaceutical Group Limited for the Greater China region to submit and commercialise Illuccix.











