island-pharma-fda Island Pharmaceuticals Ltd (ASX: ILA), an island-pharma-fda Australian based antiviral drug development firm, has had a Type C meeting with the US Food and Drug Administration (FDA) in its continued development of Galidesivir, a broad-spectrum antiviral molecule. island-pharma-fda On 29 August 2025 (US time), the FDA received a request to hold the meeting, which is a significant milestone in the regulatory journey of Galidesivir.
Engagement with island-pharma-fda FDA on Galidesivir Approval island-pharma-fda Strategy
The reason behind the meeting is to seek an agreement with the FDA on how Galidesivir should be approved using the Animal Rule. island-pharma-fda The regulatory provision provides that in situations where neither an ethical nor feasible human trial can be carried out, the FDA may accept data of animal efficacy to approve drugs, provided that human safety is confirmed and the disease is well modelled in animals.
The Type C meeting will give Island Pharmaceuticals an FDA response on documentation, study design, quality control, and the possibility of island-pharma-fda receiving a Priority Review Voucher (PRV) that may hasten the subsequent drug approvals. The company anticipates that the meeting will be held in the fourth quarter of calendar year 2025 island-pharma-fda which is within its timelines.
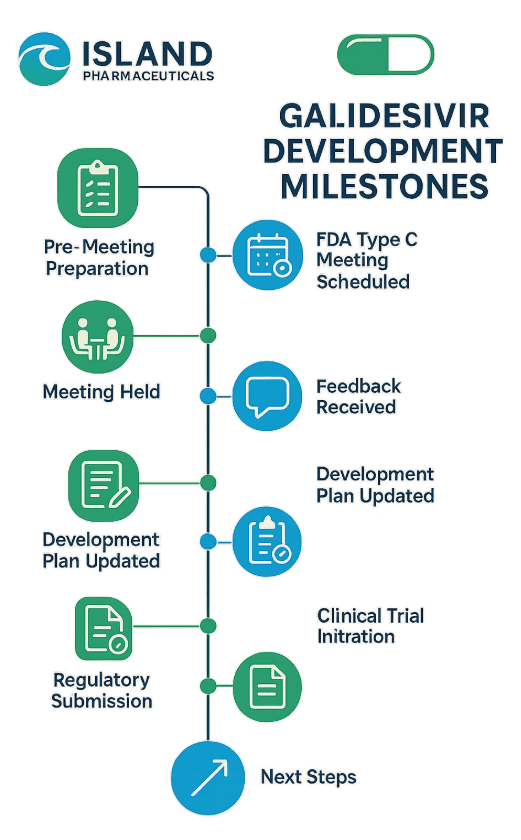
Timeline showing Island Pharmaceuticals’ Galidesivir FDA development milestones
Background on Galidesivir and Development Plans
Galidesivir was acquired by Island Pharmaceuticals from BioCryst Pharmaceuticals for $550,000 earlier this year. The island-pharma-fda antiviral has demonstrated broad activity against over 20 RNA viruses, including high-priority pathogens such as Marburg virus, Ebola, MERS, Zika, and Yellow fever. The development of Galidesivir has island-pharma-fda been supported by over US$70 million in funding from the US government, underpinning its clinical history.
Island Pharmaceuticals aims to fast-track Galidesivir’s approval to treat Marburg island-pharma-fda virus using the FDA’s Animal Rule pathway. The island-pharma-fda company is advancing an animal study designed to generate efficacy data, which remains on track to commence and complete within the next quarter.
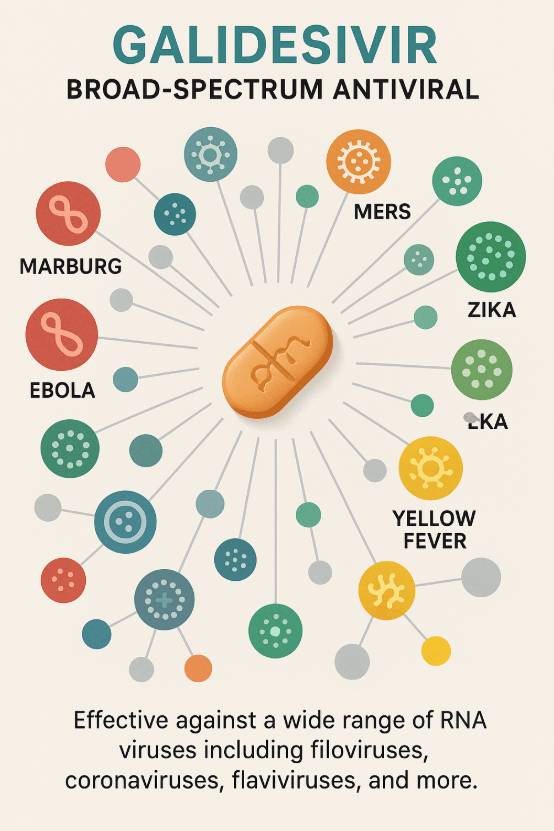
Broad-spectrum antiviral activity of Galidesivir against major RNA viruses
CEO Commentary on FDA Engagement
Dr David Foster, CEO and Managing Director of Island Pharmaceuticals, stated, “We are very pleased to have commenced engagement with FDA around the development and approval strategy for Galidesivir. This meeting will provide the opportunity to gain important feedback from the regulator and seek alignment on use of the Animal Rule to accelerate the approval process.” He added that the company continues to work with multiple sites and potential partners to identify a suitable facility to conduct the planned animal study.

FDA Animal Rule pathway for Galidesivir’s regulatory approval process
Regulatory and Market Implications
The Type C meeting offered by the FDA is a significant chance that will enable Island Pharmaceuticals to reduce risks in its development strategies and it could save time and money spent on the traditional human clinical trial procedures. This is evidenced in historical instances in which compliance with regulatory bodies using such meetings has saved millions of dollars in development expenditure as well as simplifying market entry routes.
Galidesivir projections in the market present it as a solution to major threats to the health of the populace. Galidesivir treatment has a potential market size of about US$500 million across the globe by 2033 due to government investments and the rising demand for antiviral agents in the treatment of emerging viruses.
Also Read: Trump Calls Out Putin’s Failures as Ukrainian Drones Strike Russia’s Oil Lifeline
Investor and Market Response
The market capitalisation of Island Pharmaceuticals is approximately A$90 million, and the stock of this company actively trades and its average volume is greater than 630,000 shares. This FDA meeting and similar regulatory milestones can be discussed as a potential trigger point in the valuation increase of investors interested in biotechnology and antiviral solutions in general.
Next Steps: Animal Studies to Propel Galidesivir Toward Approval
Island Pharmaceuticals’ formal FDA Type C meeting request for Galidesivir is a strategic move to advance the antiviral’s development for Marburg virus treatment using the Animal Rule. This engagement marks a key regulatory milestone that could accelerate the asset’s approval process and position the company as a provider of solutions for high-priority public island-pharma-fda health threats. The company’s commitment to meet the FDA in 2025’s fourth quarter and progress animal studies underscores its focused development timeline and regulatory strategy.
This development aligns with Island’s broader dual antiviral strategy addressing urgent viral diseases and biosecurity threats, reinforcing its standing in the island-pharma-fda antiviral drug development sector.