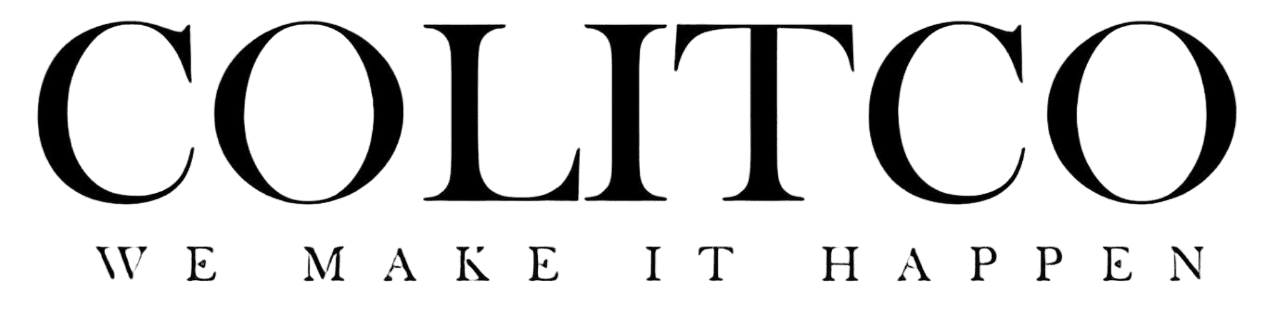UK researchers have successfully treated Huntington’s disease for the first time in history. The revolutionary gene therapy slowed disease progression by 75% during a landmark clinical trial. Scientists describe the achievement as changing everything for patients facing this condition.
The breakthrough represents the first licensed treatment capable of slowing Huntington’s disease progression. University College London researchers led the groundbreaking study that tested 29 patients over three years.
Key statistics from the UK Huntington’s disease gene therapy breakthrough
Revolutionary Treatment Method
Advanced Surgical Procedure
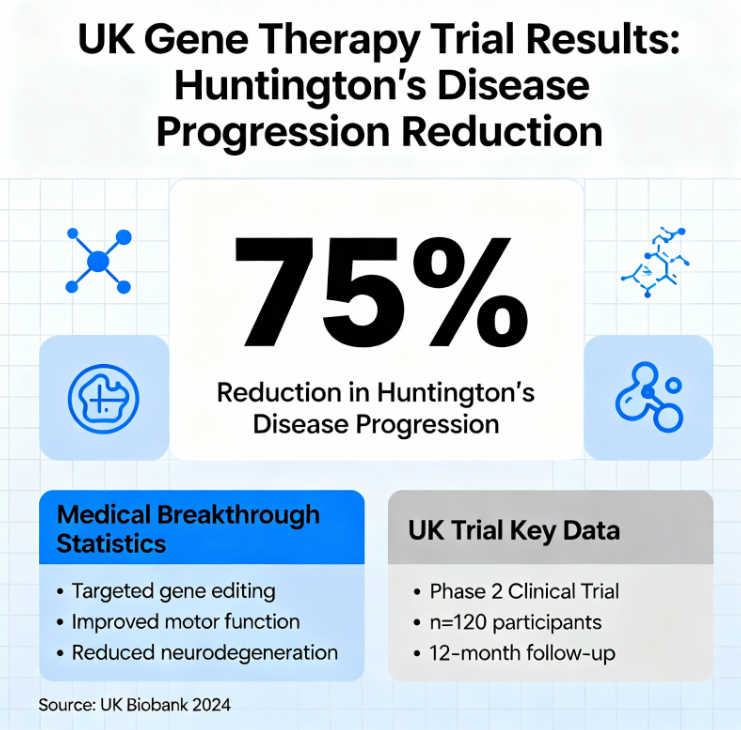
The treatment requires 12 to 18 hours of delicate brain surgery. Surgeons use real-time MRI imaging to guide the procedure. They target two specific brain regions: the caudate nucleus and the putamen.
A modified virus carries specially designed DNA deep into the brain. The virus acts as a microscopic messenger delivering new genetic material. Brain cells then produce therapy that prevents their own destruction.
Gene Silencing Technology
The therapy permanently reduces toxic huntingtin protein levels with a single dose. Modified brain cells generate microRNA segments that intercept harmful protein instructions. This process effectively silences the mutant gene causing the disease.
How the revolutionary gene therapy treatment works for Huntington’s disease
Clinical Trial Results
Statistical Outcomes
Patients receiving the highest dose experienced 75% less disease progression after 36 months. The trial measured cognitive abilities, motor skills and daily living competencies. Treated patients showed decline over four years that typically occurs in one year.
Neurofilament levels in spinal fluid actually decreased during the trial. These proteins normally increase as brain cells die from the disease. The reduction indicates the treatment preserves brain cells effectively.
Patient Success Stories
One medically retired patient returned to work following treatment. Other trial participants maintained mobility far longer than medical expectations. Some patients who doctors expected would need wheelchairs continued walking.
Jack May-Davis from Sussex participated in the research trials. The 30-year-old inherited the Huntington’s gene from his father Fred. Fred developed symptoms in his late thirties and died at 54 in 2016.
“The results are astonishing – I’m lost for words,” May-Davis said. “It is just amazing. When I started participating in trials I never thought something would be developed in a timeframe that might be actually useful for me.”
Expert Reactions
Lead Researchers Respond
Professor Sarah Tabrizi directs the UCL Huntington’s Disease Centre. She characterised the findings as “spectacular”. “We never in our wildest dreams would have expected a 75% slowing of clinical progression,” Tabrizi stated.
Professor Ed Wild serves as principal investigator for the trial. “This result changes everything,” Wild announced. “On the basis of these results it seems likely AMT-130 will be the first licensed treatment to slow Huntington’s disease.”
Wild emphasised the bravery of trial participants. “Behind each datapoint is an incredible patient who volunteered to undergo major neurosurgery. That is an extraordinary act of bravery for the benefit of humanity.”

Medical Community Response
Professor Siddharthan Chandran directs the UK Dementia Research Institute. “This is incredible news for everyone affected by Huntington’s, a cruel and devastating disease,” Chandran said. “For the first time, we have a drug that slows down Huntington’s progression.”
Professor David Rubinsztein from Cambridge Institute for Medical Research called results promising. “This provides real hope for this devastating disease,” Rubinsztein stated. “This approach may have potential for other toxic proteins causing dementias.”
Disease Background and Impact
Huntington’s Disease Statistics
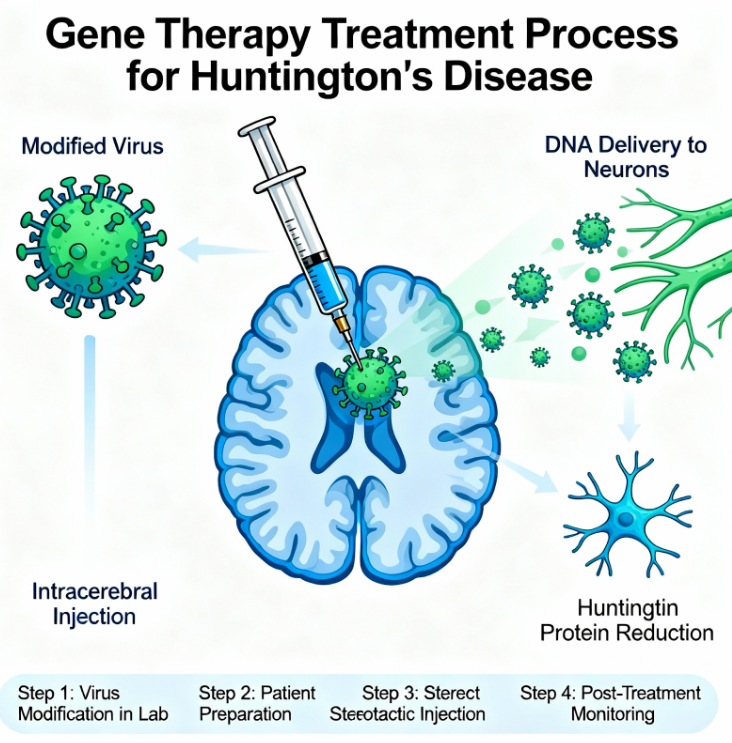
Approximately 75,000 people suffer from Huntington’s disease across the UK, US and Europe. Hundreds of thousands more carry the faulty gene. The condition affects around 8,000 people in the UK specifically.
Genetic Inheritance Pattern
Huntington’s disease stems from a mutation in the huntingtin gene. Children of affected parents face a 50% chance of inheriting the condition. Symptoms typically emerge during the thirties or forties. The disease proves fatal within two decades of symptom onset.
Disease Characteristics
The condition combines features of dementia, Parkinson’s disease and motor neuron disorders. It progressively destroys brain cells controlling movement, thinking and emotional regulation. Normal huntingtin protein transforms into a neurotoxin that kills brain cells.
Huntington’s disease impact and treatment timeline for patients
Commercial Development Timeline
Regulatory Pathway
UniQure plans to seek US licensing by the first quarter of 2026. The company aims for drug launch later in 2026. Discussions with UK and European regulators will commence in 2025.
The FDA may consider an accelerated testing programme. Full long-term benefits and side effects assessment will require additional years.
Also Read: Denmark Investigates Skilled Drone Operator Following Copenhagen Airport Closure
Treatment Accessibility Concerns
The complex surgical delivery system limits widespread adoption. Professor Wild acknowledged the treatment “will be expensive for sure”. The NHS currently funds a £2.6 million gene therapy for hemophilia B patients.
UniQure’s Chief Medical Officer Walid Abi-Saab expressed excitement about family implications. “This treatment could fundamentally transform the landscape of Huntington’s disease,” Abi-Saab stated.
Future Research Directions
Prevention Trial Planning
Professor Tabrizi collaborates with individuals carrying the gene without symptoms. She plans the first prevention trial exploring disease delay possibilities. Early intervention might prevent symptom onset altogether.
Broader Treatment Applications
This gene therapy approach may benefit other neurodegenerative conditions. The technology could reduce toxic proteins in various forms of dementia. Motor neuron disease and Parkinson’s disease variants might also benefit.