CSL Limited (ASX: CSL; USOTC: CSLLY) is stepping into a landmark moment. On 18 Feb 2026, the Melbourne-based biopharmaceutical Company announced an exclusive licensing agreement with Eli Lilly and Company. The CSL Eli Lilly partnership centres on clazakizumab, an anti-interleukin-6 (IL-6) monoclonal antibody with wide therapeutic potential.

Figure 1: CSL Limited global operations facility, highlighting the Company’s international biopharmaceutical infrastructure. [Source: CSL Limited]
The deal brings an immediate upfront payment of US$100 million to CSL. The Company is also eligible for clinical, regulatory, and commercial milestone payments, plus royalties on global net sales. This CSL ASX announcement signals a confident push into the cardiovascular and immuno-inflammatory space.
What the CSL Eli Lilly Partnership Actually Covers?
- CSL Limited retains exclusive rights to develop and commercialise clazakizumab for a single, defined indication.
- This indication is the prevention of cardiovascular events in patients with end-stage kidney disease (ESKD), where the kidneys are no longer able to function adequately without intervention.
- Eli Lilly assumes responsibility for the broader development pathway of clazakizumab beyond ESKD.
- Lilly will lead the development, global regulatory approval, and commercialisation of the therapy across additional indications.
- This structured division of rights positions the CSL pharma partnership as a well-designed dual-track development strategy, enabling both companies to maximise value within their respective focus areas.

Figure 2: Eli Lilly Biotechnology Centre signage, representing the Company’s global research and development capabilities. [Source: MedCity News]
The Science Behind Clazakizumab
Clazakizumab was originally developed by Vitaeris Inc. CSL Limited acquired the Company in 2020. It is a monoclonal antibody that targets IL-6, a signalling molecule called a cytokine. When IL-6 is overproduced in the body, it triggers chronic inflammation. That inflammation is linked to a wide range of serious conditions.
By blocking IL-6 from binding to its receptor, clazakizumab may interrupt the inflammation cascade. For ESKD patients on dialysis, this could be meaningful. This group carries an exceptionally high risk of major cardiovascular events, heart attacks and strokes among them.
The POSIBIL6ESKD Phase 3 Trial: CSL’s Clinical Backbone
CSL Limited is actively advancing the POSIBIL6ESKD Phase 3 clinical trial (NCT05485961). The trial evaluates the efficacy and safety of clazakizumab in ESKD patients on dialysis who are at elevated risk for major cardiovascular events.
This CSL ASX announcement reaffirms the Company’s commitment to that trial. The study is a cardiovascular outcomes trial, considered a gold standard in the field. Positive Phase 2b data previously showed meaningful reductions in inflammatory biomarkers among dialysis patients.
What CSL’s R&D Head Says About the CSL Pharma Partnership News
Bill Mezzanotte, EVP and Head of Research and Development at CSL, shared his perspective on the deal.

Figure 3: Bill Mezzanotte, Executive Vice President and Head of Research and Development at CSL Limited. [Source: CSL Limited]
“This agreement marks a significant step forward in our mission to bring innovative therapies to patients worldwide. Clazakizumab is a promising therapeutic candidate with the potential to significantly impact the treatment landscape for various immuno-inflammatory and cardiovascular conditions. Lilly is another patient-focused organisation, and we look forward to working with them to maximise the potential of this important medicine.”
The CSL Eli Lilly partnership is framed by both parties as patient-first. That positioning is consistent with CSL’s long-standing mission in rare and serious diseases.
Industry Outlook: Why the CSL Pharma Partnership News Targets a Growing Market
The global end-stage renal disease market is expanding fast. It was valued at US$147.7 billion in 2024 and is projected to reach US$286.5 billion by 2030, growing at a CAGR of 11.7%. Cardiovascular complications remain the leading cause of death in ESKD patients worldwide.
This CSL Eli Lilly partnership positions both companies squarely within one of the most underserved therapeutic areas in global medicine. For CSL, it is a strategic alignment of pipeline ambition with a massive unmet clinical need.
CSL Share Price and Market Response
CSL shares closed at $152.610 per share on 18 Feb 2026. The Company holds a market capitalisation of $73.52 billion. The 52-week range sits at $149.850 to $275.790 per share, reflecting the significant correction CSL shares have experienced over the past year.
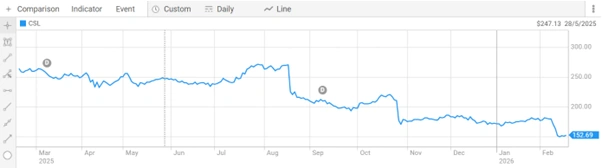
Figure 4: CSL Limited share price performance over the past year, reflecting recent market correction and investor sentiment. [Source: ASX]
This CSL ASX announcement arrives at a critical time for the stock. Analysts have widely noted that CSL is trading at a steep discount to historical levels. The Lilly deal offers a meaningful pipeline catalyst, one that could shift investor sentiment around the Company’s long-term growth story.
FAQs
Q1. What is the CSL Eli Lilly partnership about?
Ans. It is an exclusive licensing agreement. CSL grants Eli Lilly rights to develop and commercialise clazakizumab in indications beyond ESKD. CSL retains exclusive rights for ESKD cardiovascular prevention.
Q2. How much does CSL receive upfront from this deal?
Ans. CSL receives an upfront payment of US$100 million, plus potential milestone payments and royalties on global net sales.
Q3. What is clazakizumab?
Ans. Clazakizumab is an anti-IL-6 monoclonal antibody. It blocks IL-6 from binding to its receptor, which may reduce chronic inflammation in immuno-inflammatory and cardiovascular conditions.
Q4. What is the POSIBIL6ESKD trial?
Ans. It is CSL’s ongoing Phase 3 clinical trial (NCT05485961), evaluating clazakizumab’s efficacy and safety in ESKD patients on dialysis who are at risk for major cardiovascular events.