4DMedical Limited (ASX: 4DX) has sealed a game-changing distribution agreement with global healthcare giant Koninklijke Philips NV (NYSE: PHG). The expanded partnership brings a guaranteed minimum order commitment of approximately $15 million (US$10 million) over two years.
The deal positions 4DMedical’s FDA-cleared CT:VQ™ technology at the centre of Philips’ North American product catalogue. This marks a pivotal moment for the Melbourne-based medical technology company.
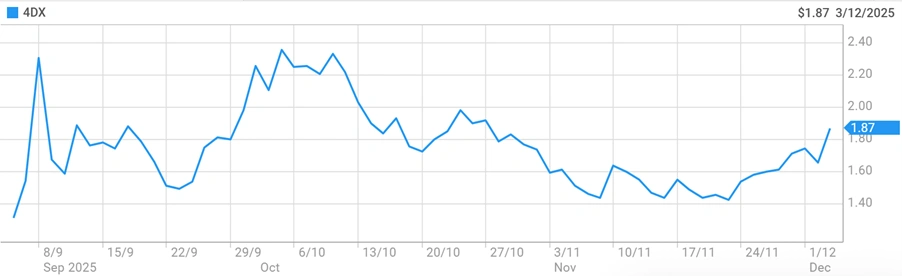
4DMedical shares surged 18% on Wednesday following the announcement, trading at $1.96 during midday sessions.
Philips Commits Resources to Drive CT:VQ Adoption
Under the expanded agreement, Philips will deploy dedicated sales and clinical specialists across the United States and Canada. These teams will carry specific CT:VQ™ sales targets as part of their responsibilities.
The announcement came during a breakfast session at the Radiological Society of North America (RSNA) annual meeting. RSNA represents the largest radiological conference in North America.
Key elements of the partnership include:
- Minimum contractual orders of US$10 million through 2027
- Full commercial terms for all CT:VQ™ sales
- Joint marketing initiatives and co-branding campaigns
- Dedicated Philips personnel focused exclusively on CT:VQ™ distribution
- Strategic milestone payments structured over 24 months
Philips brings an established commercial infrastructure spanning 14,500 CT scanner installations across the United States. This installed base provides immediate access to hospitals and imaging centres nationwide.
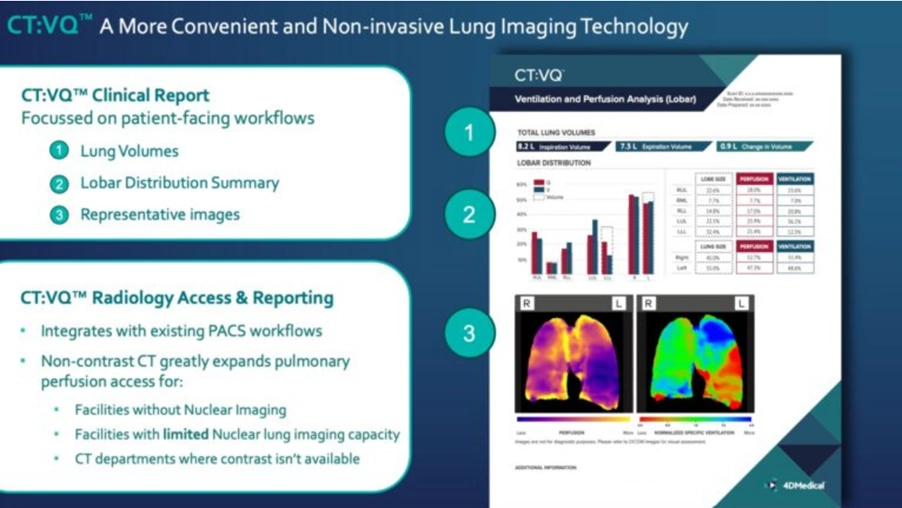
CT:VQ Technology Eliminates Nuclear Imaging Bottlenecks
CT:VQ™ represents the world’s first technology capable of extracting quantitative ventilation-perfusion data from routine non-contrast CT scans. The breakthrough addresses several critical limitations plaguing traditional nuclear VQ imaging.
Traditional nuclear VQ scans require radioactive tracers, specialised equipment, and trained nuclear medicine technologists. These requirements create scheduling bottlenecks and limit access, particularly in rural and community healthcare facilities.
CT:VQ™ bypasses these constraints entirely. The technology measures regional lung tissue motion and local density changes to generate comprehensive ventilation and perfusion maps. No radiotracers, no contrast agents, no additional infrastructure required.
The technology integrates seamlessly with existing CT protocols. Radiologists receive quantitative ventilation and perfusion data directly through their standard PACS workflows.
Clinical applications span multiple respiratory conditions:
- Pulmonary embolism diagnosis and monitoring
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) assessment
- COPD phenotyping and disease staging
- Bronchoscopic Lung Volume Reduction planning
- Tracking treatment response and disease progression
Stanford University and Brooke Army Medical Center have already integrated CT:VQ™ into their clinical workflows. The U.S. Centers for Medicare & Medicaid Services confirmed reimbursement for CT:VQ™ under Category III CPT codes.
Massive Market Opportunity Drives Growth Strategy
More than one million nuclear VQ scans are performed annually across the United States. Average reimbursement sits at approximately US$1,150 per scan.
This creates an addressable market exceeding US$1.1 billion annually in the U.S. alone. Global market potential reaches over US$2.6 billion.
4DMedical believes CT:VQ™ can rapidly capture significant market share. The company expects to eventually displace 100% of traditional nuclear VQ scans given the clinical and logistical advantages.
Management also anticipates that CT:VQ™ will drive long-term growth in demand beyond traditional nuclear VQ indications. The technology’s accessibility could expand the overall market for ventilation-perfusion imaging.
The U.S. nuclear medicine market was valued at US$6.5 billion in 2023 and continues expanding. The pulmonary imaging segment is projected to grow at a CAGR of 13.5% through 2034, driven by rising respiratory disease incidence.
Strategic Timing Aligns with Healthcare Transformation
The Philips agreement arrives just months after 4DMedical secured FDA 510(k) clearance for CT:VQ™ in September 2025. That regulatory approval opened the door to nationwide U.S. market access.
4DMedical CEO and Founder Dr Andreas Fouras highlighted the partnership’s significance during the announcement. “This agreement represents a major commercial inflection point for 4DMedical,” Fouras stated.
He emphasised Philips’ validation of CT:VQ™ as a transformative solution in pulmonary imaging. “Together with Philips, we can rapidly expand access to advanced pulmonary diagnostics, delivering powerful insights for clinicians and improved outcomes for patients,” Fouras added.

Dr Andreas Fouras, Founding CEO of 4DMedical
The partnership builds on 4DMedical’s existing relationship with Philips. Philips has distributed 4DMedical’s XV LVAS™ and CT LVAS™ products since earlier collaborations focused on U.S. Veterans Affairs healthcare applications.
4DMedical has cultivated ties with U.S. Department of Defense programs and VA medical centres. These relationships position the company strategically as respiratory health concerns grow among military veterans exposed to airborne toxins during service.
The global respiratory diagnostics market is projected to grow from $6.02 billion in 2025 to $7.24 billion by 2030. COPD, asthma, and pulmonary embolism drive sustained demand growth.
Investor Response Reflects Market Confidence
4DMedical shares demonstrated strong momentum following the announcement. The stock reached $1.96 during Wednesday trading, representing an 18% intraday gain.
Recent performance metrics show:
- One-month gain: +200% year-over-year
- Current market capitalisation: approximately $854 million
- 52-week trading range: $0.225 – $2.550
The company operates on a Software-as-a-Service model with gross margins exceeding 90%. Revenue flows through per-scan fees and subscription agreements with healthcare providers.
4DMedical’s product portfolio extends beyond CT:VQ™. The suite includes:
- XV LVAS™ for fluoroscopic lung ventilation imaging
- CT LVAS™ for regional lung function assessment
- CAC™ for coronary artery calcification analysis
- IQ-UIP™ for AI-powered interstitial pneumonia detection
- LDAf™ for emphysema and COPD mapping
The company acquired Imbio in 2023, adding advanced AI capabilities for cardiothoracic imaging. This acquisition strengthened 4DMedical’s technological foundation across respiratory diagnostics.
Technology Democratises Access to Advanced Diagnostics
CT:VQ™ leverages approximately 14,500 CT scanners already installed across U.S. healthcare facilities. This extensive infrastructure base extends advanced VQ imaging capabilities to facilities lacking nuclear medicine departments.
Rural and community hospitals particularly benefit from this accessibility. Many smaller facilities cannot justify the capital expenditure and operational complexity of nuclear medicine programs.
CT:VQ™ eliminates those barriers. Any facility with a CT scanner can offer advanced ventilation-perfusion imaging through 4DMedical’s cloud-based SaaS platform.
The technology delivers superior image resolution compared to traditional nuclear VQ scans. Quantification provides precise, objective measurements of regional lung function rather than subjective visual interpretation.
Radiation exposure from CT:VQ™ remains comparable to standard chest CT protocols. The technology requires no contrast agents or additional radiopharmaceuticals beyond the routine CT scan.
4DMedical positions CT:VQ™ as a complete replacement for nuclear VQ scanning. The company maintains this represents not just an alternative but a superior diagnostic solution across all relevant clinical indications.
What’s Next for 4DMedical and Philips Partnership
4DMedical held an investor webinar on 4th December to provide additional details on the Philips contract. The session allowed stakeholders to hear directly from leadership about commercialisation strategies.
The partnership officially launched at RSNA 2025, running from 30th November through 3rd December in Chicago. 4DMedical hosted a CT:VQ™ launch reception featuring expert radiologist and pulmonologist presentations on real clinical cases.
Philips will integrate CT:VQ™ marketing into its broader commercial programs. The medical technology giant maintains relationships with virtually every major hospital system across North America.
4DMedical continues exploring additional strategic partnerships beyond Philips. The company aims to expand CT:VQ™ availability through multiple distribution channels simultaneously.
International expansion remains on the roadmap. While the current Philips agreement focuses on North America, the global market opportunity exceeds US$2.6 billion annually.
4DMedical has raised capital through recent placements and share purchase plans. The funding supports U.S. go-to-market initiatives, clinical validation studies, and regulatory advancement in additional jurisdictions.
Also Read: Queensland Becomes Second Australian State Where Average Home Costs Over $1 Million
Frequently Asked Questions
Q: What makes CT:VQ™ different from traditional nuclear VQ scans?
A: CT:VQ™ extracts ventilation-perfusion data from standard non-contrast CT scans without requiring radioactive tracers or specialised nuclear medicine equipment. This eliminates scheduling complexity, reduces costs, and expands access to facilities lacking nuclear medicine departments.
Q: How much will Philips order under this agreement?
A: Philips committed to a minimum of approximately $15 million (US$10 million) in customer orders over a two-year period spanning 2026 and 2027. The agreement includes milestone-based payments structured over 24 months.
Q: Has CT:VQ™ received regulatory approval in the United States?
A: Yes, 4DMedical received FDA 510(k) clearance for CT:VQ™ in September 2025. The U.S. Centers for Medicare & Medicaid Services also confirmed reimbursement under Category III CPT codes, enabling nationwide access.
Q: What is 4DMedical’s current market valuation?
A: As of December 2025, 4DMedical holds a market capitalisation of approximately $670 million. The company’s shares trade on the ASX under ticker symbol 4DX.
Q: Can CT:VQ™ work with existing CT scanners?
A: Yes, CT:VQ™ integrates with approximately 14,500 existing CT scanners across the United States. The technology operates as cloud-based software without requiring hardware modifications or additional equipment purchases.












