4D Medical, an Australian medical imaging company, has achieved a historic milestone. The US Food and Drug Administration (FDA) has granted 510(k) clearance for its CT:VQ lung imaging technology. This approval marks the first time a non-contrast, computed tomography (CT)-based ventilation-perfusion imaging tool has gained FDA clearance. The clearance permits 4D Medical to commercialise CT:VQ in the United States, opening a significant market opportunity valued at over US$1.1 billion annually.
New Lung Imaging Innovation
The CT:VQ technology revolutionises lung function imaging. It evaluates both ventilation and perfusion using standard chest CT scans without requiring contrast agents or radioactive tracers. Traditional methods rely on two separate nuclear medicine scans, which take between 45 to 90 minutes and use radioactive substances. In contrast, CT:VQ produces detailed ventilation-perfusion maps in the time it takes to perform a standard CT scan. This reduction in procedure time enhances patient throughput and simplifies hospital scheduling.
The technology works by measuring lung tissue motion and changes in density to create quantitative maps of airflow and blood flow distribution throughout the lungs. These images assist doctors in diagnosing and monitoring respiratory conditions, including pulmonary embolism, asthma, and chronic obstructive pulmonary disease (COPD). The elimination of radiotracers mitigates risks associated with radiation exposure and complex handling requirements, allowing safer and broader patient access.
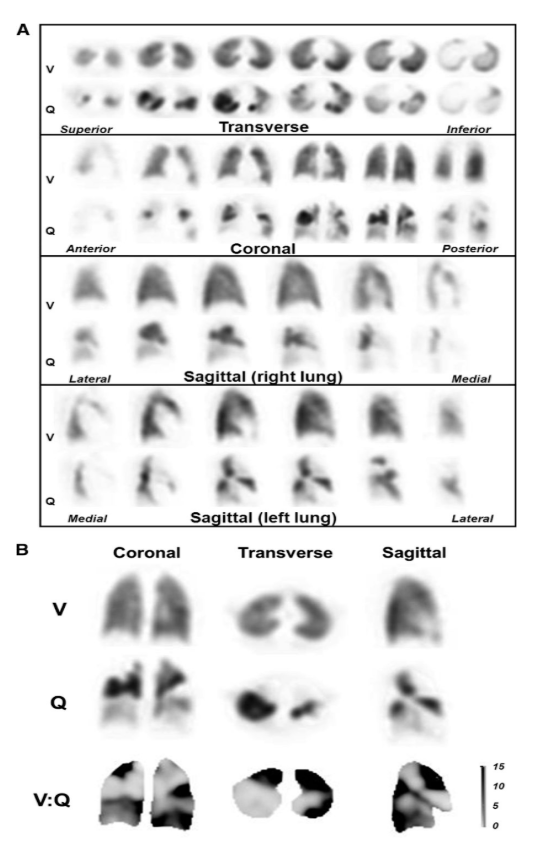
Visual of CT:VQ lung ventilation-perfusion
Clinical Validation and Adoption
CT:VQ underwent extensive clinical validation, with results benchmarked against single-photon emission computed tomography (SPECT), the current gold standard for nuclear ventilation-perfusion imaging. Comparative studies demonstrated strong agreement between CT:VQ and SPECT.
Clinicians noted CT:VQ provided clearer images and avoided artefacts that can arise from radiotracer clumping or leakage. Early adoption includes research programs at notable US healthcare institutions such as Stanford University and Brooke Army Medical Center.
According to 4D Medical CEO Andreas Fouras, the FDA clearance represents “a complete redefinition of the standard of care in pulmonary imaging” as it allows perfusion scans without exposing patients to radioactive tracers. The company plans to collaborate closely with healthcare providers and academic partners to accelerate adoption throughout the US.
Also Read: €2 Trillion Dutch Pension Shakeup Set to Reshape European Bond Markets in 2025
Market Potential and Commercial Strategy
The US performs over one million ventilation-perfusion scans annually, generating approximately US$1.1 billion in revenue. CT:VQ aims to capture a significant share of this market by replacing traditional nuclear scans.
Its compatibility with about 14,500 CT scanners already installed across the US expands the potential user base to many smaller and regional hospitals without nuclear medicine facilities. This capability democratises access to functional lung imaging.
4D Medical projects seamless integration of CT:VQ into existing hospital workflows, supported by established reimbursement rates. By removing the need for radiotracers, CT:VQ could drive increased scan orders, especially for chronic disease management and routine lung screening, expanding the overall respiratory diagnostics market. The company positions itself for rapid commercial rollout, leveraging its software-as-a-service (SaaS) business model, targeting high gross margins.
Future Outlook in Respiratory Diagnostics
4D Medical continues to focus on enhancing respiratory care through image processing and artificial intelligence. Its XV Technology platform transforms traditional lung scans into functional insights. CT LVAS software, previously FDA-cleared, complements CT:VQ by providing ventilation imaging, while CT:VQ adds combined ventilation and perfusion assessment in a single scan.
The introduction of CT:VQ aligns with global trends in respiratory diagnostics, a market forecast to reach US$7.24 billion by 2030. By improving diagnostic accuracy, patient safety, and healthcare efficiency, 4D Medical’s technology has the potential to become a new standard in lung disease detection and management worldwide.