Roquefort Therapeutics PLC (LSE: ROQ) (Roquefort or the “Company”) is a public company listed on the Main Market of the London stock exchange. Roquefort is a biotechnology company developing innovative cancer medicines in the high growth oncology market. Led by CEO Ajan Reginald, Roquefort has portfolio of four pre-clinical anti-cancer drugs and 46 registered patents.
Roquefort Therapeutic’s Portfolio
The company’s portfolio includes four best-in-class programs, Midkine antibodies, Midkine RNA therapeutics, STAT-6 siRNA and MK cell therapy, built through the acquisition of Lyramid in 2020 and Oncogeni in 2022:
Midkine (MDK) Programs:
Roquefort has two Midkine programs, developed and run by the acquired Lyramid team.
Midkine, a heparin-binding protein, has long been known to be important in embryonic development. While barely detectable in healthy adults, Midkine is highly expressed during oncogenesis. MDK hinders the normal immune response to tumours and promotes metastatic spread to other organs, thereby contributing to various levels of cancer progression and reduced patient survival.
Roquefort believes its know-how and patent strategy (the combination of the exclusive license and the efforts to patent in-house R&D) have established the entry barrier in targeting MDK. The patent strategy is focused on both the composition of matter patents and method patents for antibodies, RNA approach and truncated MDK (multiple sequences).
Management also believes that the team is one year ahead of any competition in the pre-clinical stage of developing reagents targeting the growth factor MDK for treatment of cancer.
- Midkine Antibody Therapeutic Program
Roquefort has two Midkine antibody candidates: humanised N-domain antibody (ROQA1) and the humanised C-domain antibody (ROQA2).
Both ROQA1 and ROQA2 antibody programs demonstrated significant anti-cancer activity in validated in vivo models of metastatic tumours. The results showed a significant impact in metastatic breast cancer (P<0.05) and Osteosarcoma (P<0.05) reducing both the number and size of lung metastases.
Humanised antibodies have been manufactured for both programs, with ROQA2 successfully tested in both rodent and non-human primate Good Laboratory Practice (GLP) toxicology/pharmacokinetic studies.
On the basis of these positive results and the early commercial potential to be first to market with an anti-Midkine oncology antibody, Roquefort is accelerating development of the antibody programs which remain on track for CTA/IND filing in late 2023.
- Antisense Oligonucleotide Program (Midkine RNA)
Roquefort believes that the RNA approach is highly complementary to antibodies to create an anti-cancer Midkine portfolio.
Roquefort is developing antisense oligonucleotide drugs targeting Midkine. Oligonucleotide (with short RNAs) changes and blocks the expression of Midkine. Its lead oligonucleotide drug candidates can significantly reduce Midkine mRNA levels and generate a non-functional truncated midkine that blocks cancer Midkine receptors.
In 2021, the Company entered into a collaboration with Professor Steve Wilton’s group at Murdoch University, Perth, Western Australia to design and test a novel series of gene silencing reagents, antisense oligonucleotides, targeting Midkine. Prof. Wilton is a pioneer in this gene silencing technology, having contributed to one of the FDA approvals for RNA therapeutic drugs of this style.
As announced on 11 August 2022, Roquefort announced the completion of the in vitro experiments, with its proprietary oligonucleotides successfully reducing high Midkine levels in cancer cells by generating a truncated form of the Midkine protein. The switch to the truncated MDK is consistent with the >90% efficacy at the mRNA level previously reported.
These positive results demonstrate the pre-clinical proof of principle in cancer for the antisense oligonucleotide drug development program and underpin the progress towards in vivo studies and clinical trials in due course.
Roquefort’s Mesodermal Killer (MK) Cell Program and Novel siRNAs (small interfering RNA) Program
These were both developed and are run by the acquired Oncogeni team. Both MK and siRNA families consist of four to six drug candidates each i.e., MK1-6 and siRNA 1-4. Each candidate is protected by composition of matter patents and has the potential to be a new medicine subject to the successful completion of development.
Oncogeni has two exclusive global licences to develop two families of innovative cell and RNA medicines:
- Licence Agreement with Cell Therapy Limited, the owner of three pending patent families covering intellectual property around specific Mesodermal Killer (MK) cells; and
- Licence Agreement with SIRNA Limited, the owner of eleven granted patents covering intellectual property around novel siRNAs (small interfering RNA).
Mesodermal Killer (MK) Cell Program:
Mesodermal Killer (MK) cells are a novel engineered cell type to kill cancer directly, attract Natural Killer (NK) cells and to activate (prime) natural killer cells to kill cancer. NK cells are believed to show great potential for treating cancers. However, NK cells are functionally suppressed owing to multiple immunosuppressive factors in cancer. Hence, releasing the suppressed state of NK cells by attracting and activating NK cells is a promising solution for immunotherapy. NK priming is a highly attracting field with Dragonfly / Gilead signing a $300m deal in May 2022.
MK cells were derived from the novel cellular medicine platform invented by Nobel Laureate, Prof. Sir Martin Evans’ team (2007 Nobel Laureate in Physiology or Medicine). He is currently Roquefort’s Executive Director and Group Chief Scientific Officer.
MK cells have demonstrated significantly increased cytotoxicity (P<0.05) in validated in vitro models of chronic myelogenous leukaemia and plasma cell myeloma. Incubation with MK cells significantly increased (P<0.01) the cytotoxicity of NK cells in validated in in vitro models of chronic myelogenous leukaemia and plasma cell myeloma.
The MK cells are patented and identified by a unique ‘finger print’ consisting of seven unusual receptors detectable on the surface of the MK cells (CD16, CD96, CD112, CD137L, CD178, CD253 and CD277) and the absence of three more common cell surface markers (CD34, CD45 and CD56). The seven receptors present on MKs confer key functions in direct cytotoxicity cells via contact-dependent cell lysis or antibody-dependent cell-mediated cytotoxicity (ADCC) and through the attraction and priming of NK cells.
A key advantage of the MK cells is that they are mesodermal cells, which are typically safe. There is good evidence that these mesodermal cells are safe in human subjects. Thus, MK cells are cytotoxic, but are not expected to induce any of the side effects of other cytotoxic cellular therapies, such as Chimeric Antigen Receptor-T (CAR-T) cells. In particular, MK cells are not expected to induce cytokine release syndrome (CRS aka cytokine storm), macrophage activation syndrome (MAS) and off-target effects.
Novel siRNAs (small interfering RNA) Program
siRNA, also known as small interfering RNA, is a type of non-coding double-stranded RNA of 20–23 nucleotide base pairs in length. As the name suggests, it acts by interfering with the expression of the specific gene having a complementary sequence. The siRNA binds specifically to the single gene at a particular location for gene silencing and regulation.
STAT6 siRNA act to inhibit the production of STAT6 (signal transducer and activator of transcription 6) by cancer cells. STAT6 is an intracellular target not amenable to targeting with traditional therapeutics. It is strongly expressed in various tumours and is most highly expressed in human malignant lymphomas and pancreatic, colorectal, prostate and breast cancers. Thus, techniques aimed at reducing or blocking STAT6 may be useful in treating cancers with STAT6 overexpression.
Roquefort’s STAT6 siRNA family consists of multiple patented potential siRNA medicines that have demonstrated inhibition of STAT6 production and anti-cancer activity in validated in vitro and in vivo models of breast and colon cancer. These STAT6 siRNAs demonstrated a significant reduction (P<0.01) in proliferation of both colorectal and breast with an approximately 50% reduction in cell growth at 7 days. This anti-cancer effect was replicated in a validated in vivo model of colorectal cancer with a significant reduction (P<0.05) in cancer weight and volume to 28 days. These findings suggest that silencing STAT6 could lead to better prognosis in later stages of cancer and may reduce the need for chemotherapy, and thus the side effects linked to it, while still reducing cancer size and killing the cells.
Roquefort Therapeutics’ CEO, Ajan Reginald

In picture: Ajan Reginald, CEO of Roquefort Therapeutics
CEO Ajan Reginald is a biotechnology expert with experience in drug development, biotech transactions and drug commercialisation. Before taking the CEO position at Roquefort Therapeutics, Ajan Reginald was the Global Head of Emerging Technologies at the Roche Group (SWX: ROG) and the COO and Chief Technology Officer at Novacyt S.A (LON: NCYT) and co-founded Celixir
Ajan Reginald and Prof. Sir Martin Evans, the 2007 Nobel Laureate in Medicine developed a novel cardiac cellular medicine. The medicine passed pre-clinical development and met the FDA, MHRA and EU regulatory trial approvals. Together, they formed Celixir in 2009, licensed for the Japanese market with Daiichi Sankyo, a Japanese pharmaceutical company. Celixir is valued at £220 million.

In picture: CEO Ajan Reginald and Rhodri Morgan, former First Minister of Wales
CEO Ajan Reginald is an alumnus of the Harvard Business School (AMP) University of Oxford (MSc Experimental Therapeutics), Northwestern University – Kellogg Business School (MBA) and the University of London (BDS). He has also represented England in the Masters Hockey World Cup and European Championships.
Company Financials
Roquefort Therapeutics was listed on the London Stock Exchange (LSE) in March 2021. The Company has a market capitalisation of £8.72 million. The Roquefort Therapeutics stock price has a 52-week range of 6.08-11.75 GBX. The Company has issued 129,149,998 ordinary shares of one penny per value (16th September 2022). The Company is also listed on the OTCQB Venture Market, USA.
Roquefort Therapeutics: Recent Developments
Acquisition of Oncogeni Limited
Roquefort Therapeutics acquired 100% of the shares in England-based Oncogeni Limited (Oncogenic) in October 2022. Roquefort Therapeutics raised £1.015 million by placing shares for 14 pence per share. Ajan Reginald was appointed the CEO of Oncogeni, and Prof. Sir Marin Evans was appointed the Group’s Chief Scientific officer.
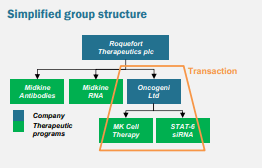
The simplified structure of Roquefort Therapeutics after the transaction
Oncogeni was founded by a Nobel Laureate and had a comprehensive product portfolio and patents created over 10+ years. The company is a spin-out of Celixir PLC. Oncogeni holds nine patents on innovative cell and RNA medicine in the pre-clinical development stage.

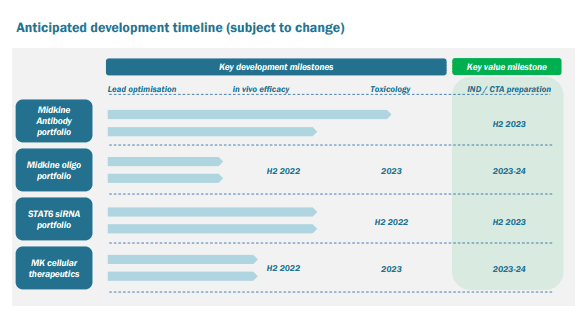
The drug development timeline of Oncogeni (June 2022)
Above is the original timeline Oncogeni followed for its drug development, which is expected to change. The tentative timeline after the acquisition is in the image below.

Expected drug development timeline after a transaction
The acquisition helped Roquefort Therapeutics achieve the following:
- It transforms Roquefort Therapeutics into a material biotech company focusing entirely on next-generation cancer drugs.
- It enhances Roquefort Therapeutics’ portfolio and adds high-value, high-growth opportunities while mitigating development risk.
- It provides Near-term IND and licensing opportunities from the advanced development stage.
- The leadership brings experienced individuals, including Nobel Laureates, into the fold and provides access to state-of-the-art laboratories.
- It provides Roquefort Therapeutics access to an international institutional investor base through Oncogeni, including Big Pharma, venture capitalists and biotech-specialised funds.
An Overview of Roquefort Therapeutics
Roquefort Therapeutics is a UK-based cancer-focused biotechnology company. The Company is led by Ajan Reginald, a leader experienced in drug development and commercialisation. the company has access to research on Midkine, a protein detected in cancer patients and those with immune and inflammatory disorders.
If you wish to learn more about Roquefort Therapeutics, visit their official website.
Disclaimer